Coronavirus Disease 2019 (COVID-19) can induce an extreme immune response characterized by the overproduction of immune cells and the uncontrolled release of pro-inflammatory cytokines. Exceedingly high levels of the cytokines IL-1, IL-6, IL-8, TNF-a, and CRP result in overt inflammatory symptoms that include mild to severe respiratory disease, high fever, and cough. In progressed disease this “cytokine storm” will circulate throughout the body to trigger a surge of active immune cells into the lungs resulting in acute lung injury and acute respiratory distress syndrome (ARDS) and may be particularly severe in immune compromised subjects such as the elderly, diabetics, and those with cardiovascular disease.
This association between infection and progression of disease with inflammation suggests a strategy that would partner anti-infective agents, such as anti-virals and vaccines, with anti-inflammatory agents. An anti-inflammatory agent would be expected to mitigate symptoms including fever, pain, and swelling. Furthermore, an anti-inflammatory regimen initiated at a relatively early stage in disease progression might be expected to stem the immune over-response and to slow or even prevent progression of symptoms leading to lung injury and ARDS. Importantly, an appropriate anti-inflammatory intervention should not result in abnormal immune suppression but should target healthy immune homeostasis.
An anti-inflammatory treatment that resulted in the decrease of inflammatory cytokine signaling would seem a promising approach. Chinese researchers have identified IL-6 as a main driver of immune overreaction in COVID-19 patients and have already included elevated IL-6 levels as a biomarker of disease worsening. China’s National Health Commission has updated its treatment guidelines for COVID-19 to include Roche’s injected biologic, Actemra (tocilizumab), an inhibitor of the IL-6 receptor. Actemra, first approved by the U.S. FDA in 2010 for rheumatoid arthritis, can now be used in China to treat serious coronavirus patients with lung damage.
The Potential Role of Astaxanthin
In pre-clinical and clinical studies, astaxanthin has demonstrated the ability to decrease levels of pro-inflammatory cytokines IL-1, IL-6, TNF-a, and CRP in multiple models of disease and in several patient populations. Furthermore, astaxanthin does not lead to abnormal immune suppression even at high doses and acts to restore healthy immune homeostasis. Astaxanthin has also demonstrated exceptional safety in multiple animal models, has been used extensively in humans for two decades as a dietary supplement, and is GRAS (Generally Recognized as Safe), according to FDA regulations.
Coronaviruses have been shown to induce lung damage by increasing inflammatory signaling pathways and cytokine production leading to elevated immune cell infiltration and macrophagic polarization shifts (M2 to M1). Astaxanthin has been shown to (i) significantly attenuate pathological elevation of critical inflammatory cell signaling pathways (NF-kB), (ii) decrease the resulting elevated proinflammatory cytokine levels, (iii) reduce immune cell infiltration of the lung, and (iv) positively influence macrophage polarization in humans and animal models of disease. (Further discussion and references are provided in the white paper, available for download.)
Results from the pre-specified interim review of the Company’s ongoing CHASE (Cardiovascular Health Astaxanthin Supplement Evaluation) clinical trial demonstrated a statistically significant reduction in CRP (C-Reactive Protein, a measure of inflammatory health) in diabetics and a strong trend of CRP reduction in cardiovascular patients. The CHASE clinical trial is a double-blind, randomized, placebo-controlled clinical trial evaluating the effect of the Company’s synthetic astaxanthin dietary supplement ZanthoSyn®, on cardiovascular health, as measured by C-Reactive Protein or “CRP” levels over 12 weeks in up to 120 subjects with documented cardiovascular risk factors. Pre-specified secondary cardiovascular/inflammatory health markers, safety parameters, exploratory endpoints, and pre-specified sub-groups are also being assessed. The trial includes an optional open-label extension through 48 weeks.
Collaboration Opportunities
Cardax is seeking strategic collaborations with appropriate academic, governmental, and/or commercial organizations to further develop astaxanthin for COVID-19. Please contact us to learn more.
Important Notice
These statements have not been evaluated by the Food and Drug Administration. Astaxanthin is not intended to diagnose, treat, cure, or prevent any disease. If you have any questions or concerns about your health, you should always consult a qualified healthcare professional. Do not disregard, avoid, or delay obtaining medical advice from a healthcare professional.
About Astaxanthin
Astaxanthin is a potent and safe anti-inflammatory that can improve the health in humans and animals. Astaxanthin is proven to reduce inflammation at its source, without the harmful side effects of common anti-inflammatories and pain relievers. Astaxanthin has undergone extensive safety testing and has a long history of use in humans and animals.
Astaxanthin has demonstrated efficacy in models of inflammatory-mediated disease, including:
- Reduction of TNF-α levels equivalent to a steroid
- Reduction of cholesterol levels
- Reduction of elevated triglycerides
- Reduction in blood clot formation with no increase in bleeding
- Decrease in myocardial tissue damage following experimentally-induced myocardial infarction
- Reduction of liver enzymes and liver histological damage
2,000+ peer reviewed papers featuring astaxanthin have been published in established scientific journals.
History and Background
Astaxanthin in Nature
Astaxanthin is widely and naturally found in marine organisms, including microalgae, crustaceans, salmon, and trout. It’s the substance that gives these marine animals their distinct color.
Animals have adapted to exploit the health benefits of astaxanthin. Astaxanthin is believed to protect microalgae from environmental stress. Salmon accumulate astaxanthin from their diet and store it in their muscle tissue; astaxanthin is believed to protect salmon from oxidative stress when they swim upstream to spawn – a taxing and traumatic event.

Identification and Study

Richard Kuhn
In 1938, Richard Kuhn identified and isolated astaxanthin from lobster. Kuhn had setup a laboratory on the banks of the Neckar River in Germany and quickly established his reputation as a leading experimentalist in organic chemistry. Astaxanthin was one of the earliest carotenoids to be examined by the broader research community.
Noting that astaxanthin was found to improve animal health and vitality, further scientific study ensued. To date, over 2,000 peer-reviewed papers exploring astaxanthin have been published in scientific journals. More than 50 peer-reviewed papers have been published by Cardax team members, ten of which appeared in The American Journal of Cardiology.
Supplementation
Humans have been consuming a diverse array of sea life, including astaxanthin, for many thousands of years, but only recently have we been able to reap the benefits of astaxanthin through supplementation.
Agricultural production of astaxanthin typically comes from the following sources:
- Krill: ~120 PPM astaxanthin
- Arctic shrimp: ~1,200 PPM astaxanthin
- Yeast (phaffia yeast): 10,000 PPM astaxanthin
- Microalgae (Haematococcus pluvialis): ~40,000 PPM astaxanthin

Safety
Commonly used anti-inflammatory drugs such as aspirin, ibuprofen, naproxen, COX-2 inhibitors, corticosteroids, and various biologics have risks of side effects including gastrointestinal bleeding, heart attacks, strokes, and severe infections. Prescription fish oil drugs, while safer than common anti-inflammatory drugs, also have risks of certain side effects. Lovaza and other DHA, EPA combination fish oil drugs, have risks of side effects including back pain, eructation, dysgeusia, and increases in LDL cholesterol. Vascepa has risks of side effects including arthralgia, atrial fibrillation, and increased bleeding. Fenofibrates have risks of side effects including stomach pain, nausea, and back pain.
In contrast, astaxanthin has no known side effects of clinical significance. We believe astaxanthin’s excellent safety profile will be a key competitive advantage compared to other drugs targeting inflammation and lipids.
Astaxanthin is Generally Recognized as Safe (“GRAS”) as a food substance according to U.S. FDA regulations and has undergone extensive toxicity testing by third parties and us with no clinically meaningful issues even at extremely high doses.
Extremely High Dose Toxicity Testing
Mechanism of Action
The mechanism of action of astaxanthin is quite different than most drugs, and we believe is responsible for its excellent safety profile.
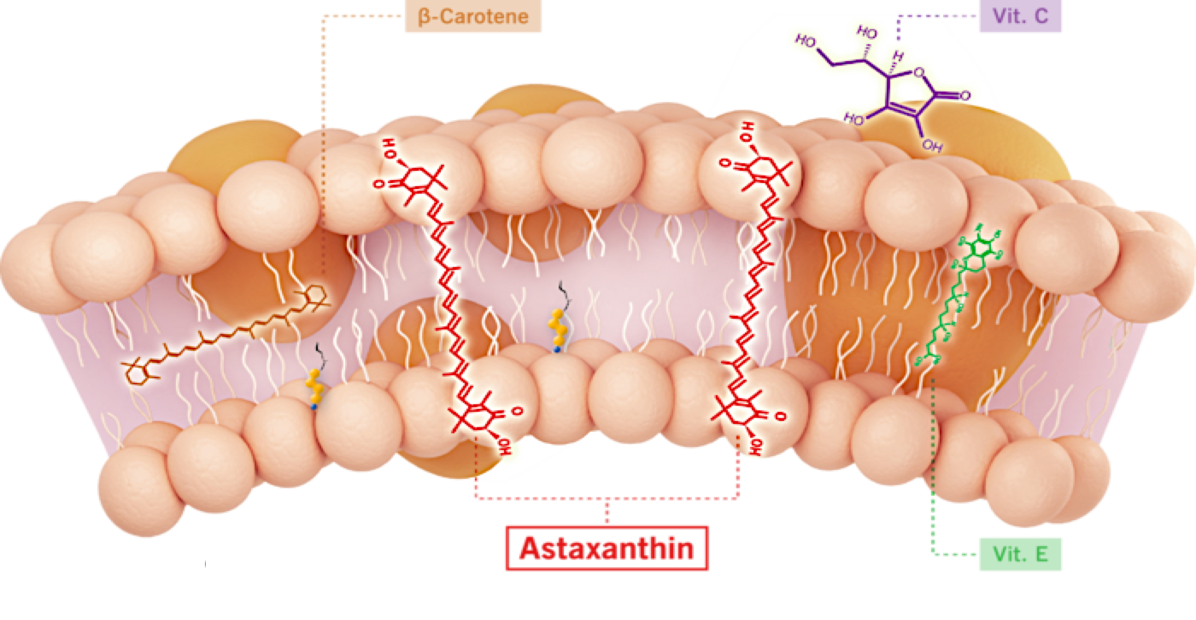
Most drugs target single receptors or enzymes in complex pathways, which can lead to side effects with chronic use. Astaxanthin is distributed systemically, including to the liver and heart, where it localizes in cellular and mitochondrial membranes and reduces the oxidative stress that causes chronic inflammation, without affecting the normal function of inflammatory/metabolic signaling pathways.
Unlike other antioxidants such as beta-carotene, Vitamin C, and Vitamin E, astaxanthin spans and stabilizes cellular and mitochondrial membranes (biological lipid bilayers) to function as an aqueous and lipid phase antioxidant without membrane disruption, as proven by X-ray diffraction studies. As a result, astaxanthin demonstrates positive and quantifiable pleiotropic effects on many inflammatory cytokines and drug targets.
Astaxanthin Reduced TNF-α in an Infllamatory Animal Model Compared to Prednisolone
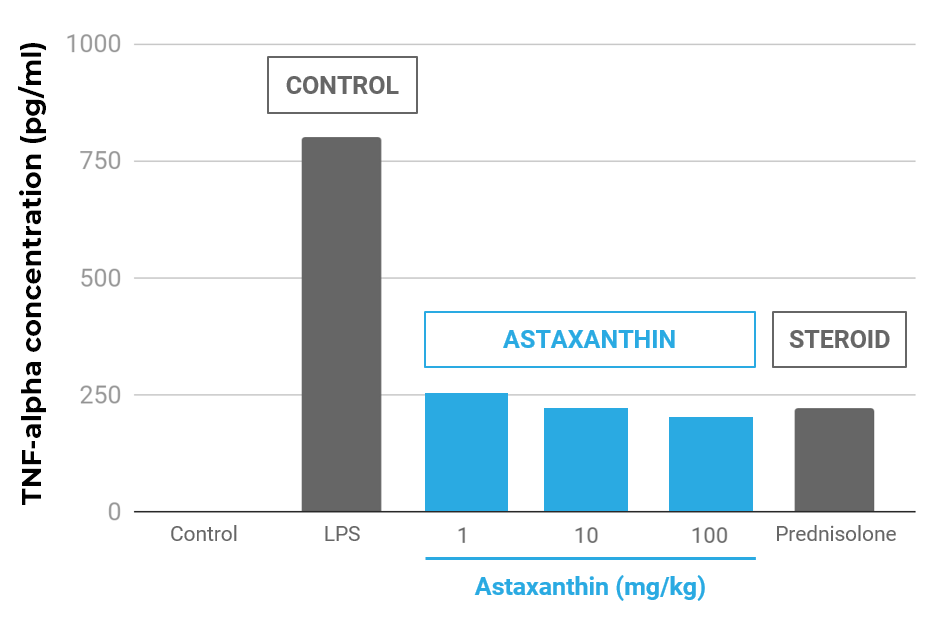
Effect of astaxanthin on TNF-α concentrations in the aqueous humor. The aqueous humor was collected 24 hours after LPS treatment. Each value represents the mean ± SD (n=8). The dose of prednisolone was 10 mg/kg. p<0.01, compared with the LPS group.
In human proof-of-concept “pilot” studies with astaxanthin conducted by third parties:
- TNF-α was significantly reduced (-30%, p=0.0022)
- C-Reactive Protein (“CRP”) was significantly reduced (-20%, p<0.05; two studies)
- Oxidative stress was significantly reduced (MDA, IsoP, SOD, TAC increased)
In animal studies with astaxanthin conducted by third parties:
- Inflammatory markers were reduced in various model systems
- TNF-α, IL-1β, IL-6, CRP, NF-kB, PGE-2, iNOS, MCP-1, ERK, JNK, COX-2
- TNF-α reduced equivalent to an equal dose of prednisolone
- Pathway inhibition (NF-kB) and activation (PI3K/AKT, adiponectin)
- Oxidative stress reduced in mitochondria
Manufacturing: Synthetic versus Natural Astaxanthin
Dietary supplements containing astaxanthin typically derive astaxanthin from microalgae, krill, or other natural sources, whereas Cardax astaxanthin is made through total synthesis. While multiple studies demonstrate that astaxanthin from either natural or synthetic sources is efficacious and both are Generally Recognized as Safe (GRAS) according to FDA regulations, we believe synthetic astaxanthin offers significant advantages compared to astaxanthin from microalgae, krill, or other natural sources:
- Synthetic astaxanthin can be formulated for superior bioavailability.
- Synthetic astaxanthin has been extensively tested in a wide range of toxicity studies, including acute, sub-acute, sub-chronic, and chronic toxicity studies, carcinogenicity studies, genotoxicity/mutagenicity studies, and developmental and reproductive toxicity studies; whereas to our knowledge microalgal or other sources of astaxanthin have not undergone the same amount of safety testing in such toxicity studies.
- Synthetic astaxanthin is manufactured with superior purity and precision, whereas astaxanthin extracted from microalgae and krill oil is obtained in a complex mixture, which may include many unknown marine byproducts.
- Synthetic manufacture of astaxanthin is scalable, whereas we believe the ability to readily scale the production and extraction of astaxanthin from microalgae or other sources will be limited as demand for astaxanthin grows.
- Synthetic manufacture of astaxanthin emits fewer greenhouse gases and consumes less energy, raw material, and land than traditional microalgal astaxanthin production.

